By being certified ‡ in multiple quality processes, Excel Translations is able to work closely with our life science and corporate customers to provide language services that are compliant with the strict regulatory standards of the medical device, biotech, and pharmaceutical industries.
The Advantages of working with a Certified‡ Translation Company
ISO 9001:2015 Certified‡ Translation Company
Advantages at a Glance
Excel Translations’ medical translation services and technical translation services are performed in a quality management system that is certified‡ to the 9001:2015, 13485:2016 and 17100:2015 standards. The following key ISO 9001:2015 sections govern our quality management system:
Leadership & Management Responsibility
Our vision, policies and strategic objectives are built around, among others, the following translation management principles:
- Customer Focus
- Process Approach
- Continual Improvement
Planning & Resource Management
Excel Translations allocates and directs its resources – personnel, infrastructure, information, partners, finances – for the operation and continuous improvement of its production process, as well as the full satisfaction of its customers.
Operation
The Quality Management System we’ve designed ensures the effective and efficient operation of linguistic and supportive processes, providing the quality guidelines necessary to satisfy our customers and to ensure that we are a certified‡ translations company.
Performance Measurement, Analysis & Improvement
Measurement data are important in making fact-based decisions. We can ensure the effective and efficient measurement, collection and validation of data to ensure its performance and the satisfaction of its customers.
ISO 13485:2016 Certified‡ Translation Company
“Due to compliance implications for the essential requirements of the MDD and the IVDD, Notified Bodies consider translation to be an ‘important outsourced service’…This makes translation providers subject to the outsourced vendor risk management considerations of ISO 13485 and ISO 14971.” – KEMA
ISO 13485:2016 Advantages
We are certified‡ to the ISO 13485:2016 standard, a quality management system, which details requirements for companies that manufacture medical devices and related services.
It is important to note that the requirements of ISO 13485:2016, which are applicable to medical devices, also specify that work performed in conjunction with the medical device or service by an outside vendor remains the responsibility of the medical device organization.
Therefore, by working with an ISO 13485:2016-certified‡ translation company, medical device organizations can reduce costs and lower risks regarding Notified Body Inspections, and likely minimize the number of non-conformities during the audit process.
Below are some of the primary reasons why more and more medical device companies opt to outsource their medical translations to a certified‡ translation company.
QMS Harmonization
The quality system of the medical device manufacturer and the quality system of the ISO 13485:2016 Certified‡ Translation Company will be adhering to the same requirements or, if you will, will be ‘speaking the same language’ (pun intended.)
Audits
By outsourcing medical translations to an ISO 13485:2016 Certified‡ Translation Company, medical device manufacturers can improve risk mitigation during their ISO 13485:2016 audits.
Translation Vendor Qualification
Medical device manufacturers can introduce a shorter qualifying process and significantly reduce their resource allocation for supplier qualification and control.
ISO 17100:2015 Certified‡ Translation Company
In addition to its ISO 9001:2015 and ISO 13485:2016 certifications, Excel Translations is certified‡ to the ISO 17100:2015 standard. ISO 17100 provides requirements for the core processes, resources, and other aspects necessary for the delivery of a quality translation service that meets applicable specifications.
A Standard Specifically Designed for the Translation Industry
ISO 17100:2015 is a standard specifically designed with the translation industry in mind, including life sciences translation. It covers all aspects of the translation process and defines basic parameters for a quality translation process. ISO 17100:2015 demystifies and explains the critical components associated with the translation process.
ISO 17100:2015 aims to standardize terminology, define basic requirements for services, and create a framework for interaction between customers and services providers.
Identify Requirements and Evaluate Suppliers
ISO 17100:2015 offers clients guidance to the services offered by translation service providers, especially for those companies that are new to translation or about to enter a foreign market.
Benchmarking and Assessment Tool
ISO 17100:2015 also provides clients with a reference point (a benchmark) for quality. The standard is widely used as a tool to evaluate Translation Service Providers and assess their quality performance.
Confidence
By working with a translation company that is certified‡ to ISO 17100:2015 and competent in life sciences translation, you, your customers and other stakeholders can have confidence that the translated documents and software that accompany your products are meeting quality and service requirements that the translation industry believes to be necessary for the successful completion of a translation.
The ASTM F2575 Standard Guide for Quality Assurance
Excel Translations has incorporated the recommendations and guidelines of ASTM F2575 into its ISO 9001:2015, ISO 13485:2016, and ISO 17100:2015 certified‡ Quality Management System. This Standard Guide for Quality Assurance in Translation identifies factors relevant to the quality of language translation services for each phase of a translation project. The prevailing idea behind ASTM F2575 is cooperation between the client and the translation service provider, with the goal of successfully meeting the client’s requirements. Furthermore, ASTM F2575 offers an educational tool that provides hands-on information and “how-to” guidance to educate buyers of document translation services. The guide helps them facilitate communication and reach established goals in timing, cost and quality during the life of a translation project.
Road Map
ASTM F2575 serves as a road map and itemizes a sequence of steps that will increase the chance to obtain high-quality translation products and services.
- Selecting a translation service provider
- Defining project specifications
- Production (Terminology Management, Translation, Editing, Formatting, Proofreading, and Quality Control)
- Post-project review
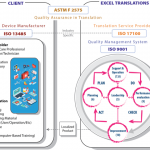
The graphic above (click to enlarge) describes how and where the various standards come into play between Excel Translations and a device company, utilizing the ASTM F2575 Standard Guide.
Quality Assurance Processes

Excel Translations has established, documented, and maintains Quality Management System to ensure a quality program that supports its efforts to provide customers with quality products and services. Excel Translations’ quality management strategy is based on the following principles:
- We maintain a documented Quality Management System designed to meet the requirements of ISO 9001:2015, ISO 13485:2016 and ISO 17100:2015. It is through the implementation of this Quality System that our Quality Policy commitments are realized.
- Through the use of documented and repeatable processes, quality plans, corrective and preventative actions, internal audits and effective management reviews, Excel Translations strives to provide the necessary tools and skills for its employees to continuously make improvements in the quality and consistency of products and services.
Quality System Documents
- The procedures and documents that make up the Quality Management System are defined and described in the Excel Translations Quality Manual.
- These documents collectively define a Quality System that complies with ISO 9001:2015, ISO 13485:2016 and ISO 17100:2015 and identifies the methodology for implementing the company’s quality policy commitments.
Quality System Implementation
- All personnel who manage, perform, and verify work that affects quality are responsible for implementing the company’s quality system.
- Implementation of the quality system is assessed regularly by internal and external audits and regularly reviewed by management.
Quality Planning
- Our requirements for quality planning are met by documenting specific procedures, practices, tasks and activities for all production and production-supporting processes. Implementation of and adherence to these documents guarantee quality products and services. The quality documentation includes meeting contractual requirements, and understanding and meeting customer needs – with an emphasis on problem prevention.
- Changes to the quality system are made as needed.
Control and Supervision
At the heart of our Quality Assurance Process is a double-check mechanism. Simply put, every action is double-checked by a second person, following defined procedures and authorization levels. Though deceptively simple, the logistics of organizing a double-check procedure are daunting.
For example, a nine-language project containing three documents may undergo seven actions. That totals 189 discreet tasks (9 x 3 x 7) to be performed and checked! Excel Translations’ process builds in a sophisticated infrastructure to organize this effort, ensure completion of each action, and to collect data, thereby assuring that the final deliverable matches the expectation set forth in the initial project planning.
We make certain that our certified‡ quality systems are appropriate for every client’s needs. If necessary, we can also customize our project approach to better meet specific customer requirements.
‡ Our US HQ in Florida and our European HQ in Bilbao, Spain are both certified by TÜV SÜD America. Regardless of where your projects will be handled, we will adhere to and replicate the same quality standards. Please contact your project manager or your sales associate for further information.